3 months ago when @sco initiated his #myshittiestmethod challenge. Back then I loudly thought about adding my own special lab experience to this challenge too.
Well, however, I didn't manage to do it on time. Maybe due to curiosity whether this would be the right platform to talk about it. Therefore a disclaimer: everything of the following is my own opinion on what happened.
A side entry to the challenge, for his and maybe someone else's amusement.
Some basics
I wrote my master thesis in physics in 2008-2009. After changing from theoretical solid state physics to a group doing experimental solid state physics a new topic was found soon: ionic conductors, or, to be more specific: the conductivity scheme of calcium fluoride. Similar investigations had been done before with barium fluoride single crystals and a polycrystalline version of calcium fluoride. My task would be to examine the conduction of a single crystal of calcium fluoride.
I was still in love with theory so there would be a large theoretical part too. Or should have been as you will see later.
We took into account 3 measurement methods: 2 NMR based ones and impedance spectroscopy. I will limit the scope of this article to the impedance spectroscopy part.
Calcium fluoride is an ionic conductor with a cubic lattice structure built by a fcc lattice of calcium ions and a smaller sc lattice of fluorine ions (Picture from Wikipedia, no copyright reserved).
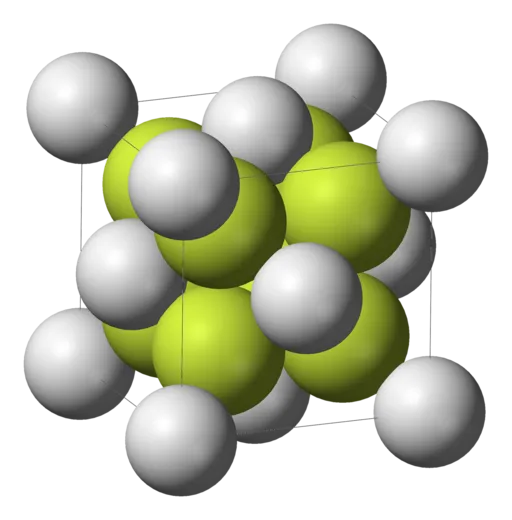
Both types of ions may move within the lattice due to the intrinsic Anti-Frenkel defect (which means that anions go interstitial) and by application of an electric alternating current or an increase of temperature, the conduction increases too.
In fact, there's an response which can be measured via NMR and via impedance spectroscopy.
Impedance spectroscopy
From a mathematical point of view the impedance is a complex function of the frequency of an alternating current.
From an engineer's point of view it's a measure for the electrical response on that alternating current. The frequencies for the current range from 5-10 Hz to up to 13 MHz, in my case 10 Hz - 13 MHz.
This is how the apparatus looked like when in use:

We collected data for the real and the imaginary part of the impedance. So-called equivalence circuits are being used as models for the temperature-dependent conductivity. Yes, the above mentioned frequency range was covered for a variety of temperatures between 600 K and 1173 K, starting from the upper temperature level. Which means: each measurement session took several hours, at least 2 for the heating of the apparatus with the sample and about 4 for the measurement. When one temperature was done, the new temperature had to be selected and the experimenter had to wait about 10 minutes till the cooling had done its job. (I had a productive time sewing some patchwork pieces by hand.)

When all temperature data had been taken, the entire system had to cool down completely. I never left before this was the case. Another 3 hours actually ... and short nights for me because I mostly did the measurements at afternoon, evening and night time when nobody could interfere.
With the equivalence circuits I determined the conductivity for each temperature - and from the conductivities I determined the activation energy for each measurement cycle - there were 6 for each sample. And this is where the "shittyness" starts.
An accident
Intentionally the measurement should have been done with one single sample. It had electrodes of platinum. It was intended to change them after the second measurement session. The process required to remove the old electrodes (I don't recall how) and to apply heated platinum to the crystal plate. The platinum was in a graphite container which accidentally exploded. Terrible!
We actually continued to use this sample for the measurements without replacing the electrodes another time. However, the results (to be seen on the left of the following graph) did not look too convincing. Where should have been two gradients there was only one. The data seemed to be useless for our theory.
After some argument it was decided to create another sample with which the entire impedance measurements would be repeated.
I probably was already behind time but which choice did we have?
The real issue
We allowed ourselves to take a little less time for the second measurement cycle - which also meant: less resting time for the sample between the sessions.
And what would happen? Again the curves (on the right) tended to have one gradient only after the second session.
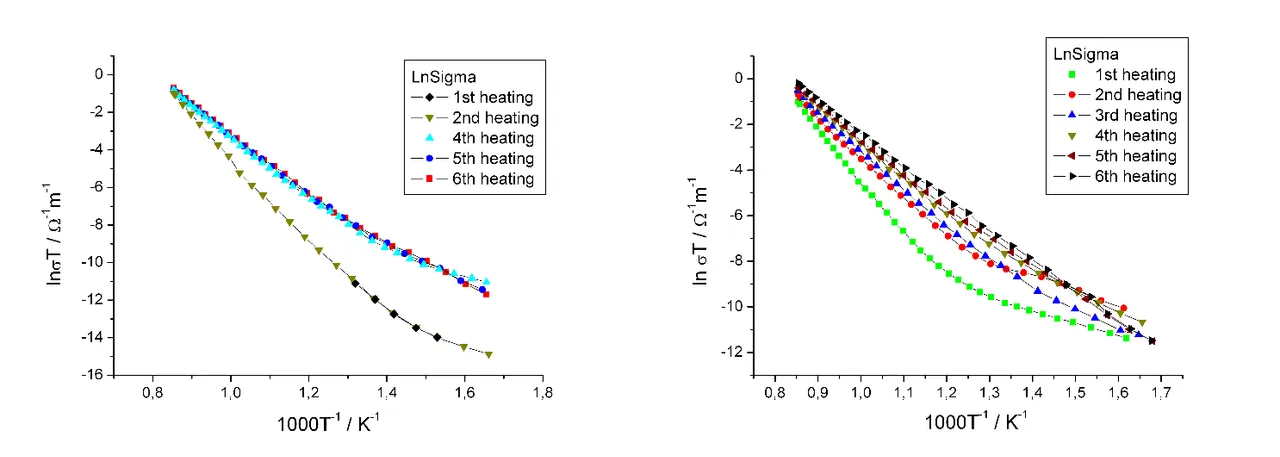
We expected two gradients for two different activation energies: one for intrinsic defects and one for extrinsic defects. Nobody could tell me what the single activation energy was about. I searched literature but came to no conclusion what really was going on here. Some kind of aging, but why and how? And what did it mean for the crystal?
I might just have accepted and left it at that. But I didn't. I shared the room with a PhD candidate who was some kind of supervisor for me. I talked him into questioning the aging of the crystal samples. Finally he agreed to talk to a colleague from the chemistry department about examining an equally treated sample by x-ray powder diffraction. The results of an untreated sample (blue) were according to theory (green) and the results for the treated (red) sample were not. Pity?
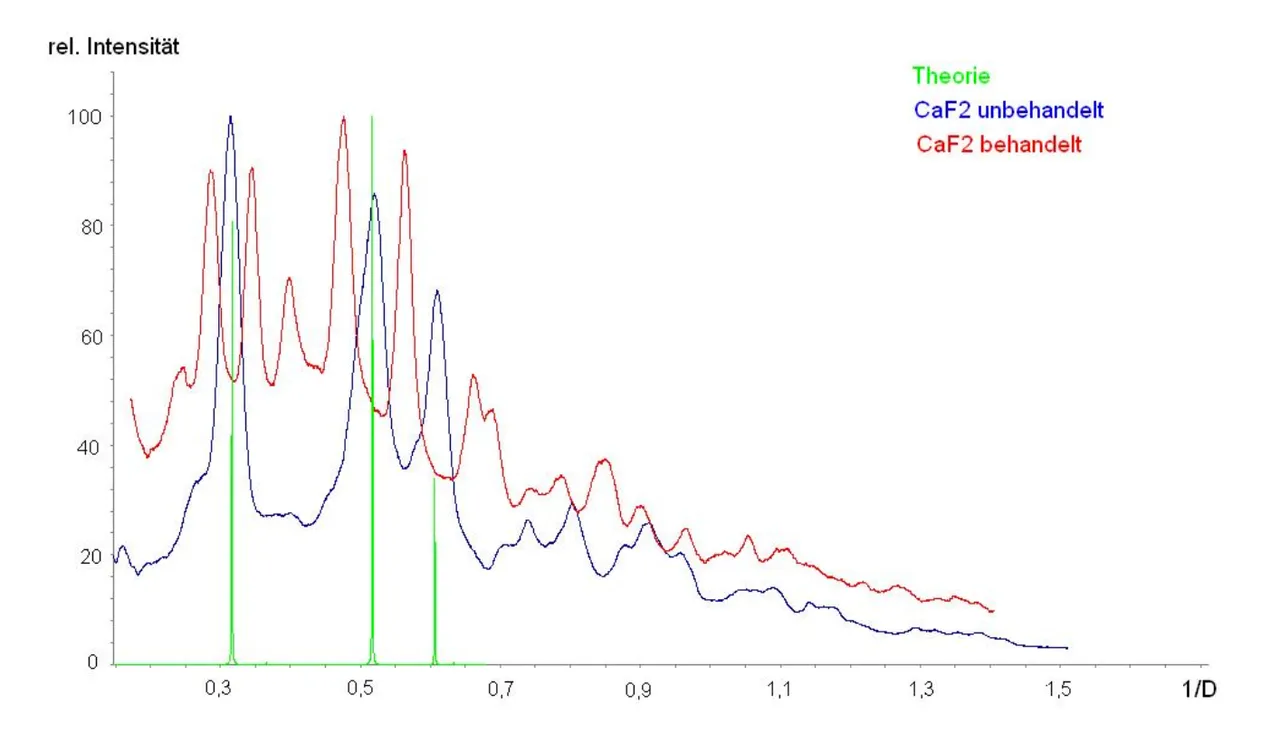
Later, NMR results showed that there might be some new permanent order in the crystal. Something which - if I recall correctly - had neither been seen in barium fluoride or in polycrystalline calcium fluoride.
Even more later, when I did the interpretation of the data, I failed to get results which matched the expectations which caused quite some trouble.
I was given grade 3 for the thesis which actually "not good". A black eye for me, so to speak. But I was too glad about having it done to argue. During that time another student got grade 5 ("not passed") for his thesis. Those events are rare but they happen too.
I don't know whether the data have been used later. Within the next 3 years no more ionic conductor stuff had been done as far as I know. In fact, it had only been a small side project anyway. A thesis project designed for me. And I, with my lack of experience in that field, had failed it.
This is the nature of physics. You might have thought about things as intense as you could. And your measurements might fail though. Good physics needs time and experience and reason.